In a Sealed Test Tube After the Fourth Phase Growth the Cell Number Increased Again Because
- Research article
- Open up Admission
- Published:
Shewanella oneidensisHfq promotes exponential phase growth, stationary phase civilization density, and cell survival
BMC Microbiology volume 13, Article number:33 (2013) Cite this article
Abstract
Background
Hfq is an RNA chaperone protein that has been broadly implicated in sRNA function in bacteria. Here nosotros describe the construction and characterization of a nothing allele of the cistron that encodes the RNA chaperone Hfq in Shewanella oneidensis strain MR-i, a dissimilatory metallic reducing bacterium.
Results
Loss of hfq in S. oneidensis results in a variety of mutant phenotypes, all of which are fully complemented by addition of a plasmid-borne copy of the wild type hfq cistron. Aerobic cultures of the hfq∆ mutant grow more slowly through exponential phase than wild type cultures, and hfq∆ cultures accomplish a last cell density in stationary phase that is ~2/3 of that observed in wild type cultures. We have observed a similar growth phenotype when the hfq∆ mutant is cultured under anaerobic conditions with fumarate as the terminal electron acceptor, and we take found that the hfq∆ mutant is defective in Cr(VI) reduction. Finally, the hfq∆ mutant exhibits a striking loss of colony forming units in extended stationary phase and is highly sensitive to oxidative stress induced by HtwoO2 or methyl viologen (paraquat).
Conclusions
The hfq mutant in Southward. oneidensis exhibits pleiotropic phenotypes, including a defect in metal reduction. Our results likewise suggest that hfq mutant phenotypes in Due south. oneidensis may be at least partially due to increased sensitivity to oxidative stress.
Background
Hfq is an RNA chaperone broadly implicated in sRNA function in many bacteria. Hfq interacts with and stabilizes many sRNAs, and it is thought to help promote sRNA-mRNA target interactions [ane, 2]. Hfq protein monomers grade a homohexameric ring that is thought to be the most agile form of the poly peptide [3, iv]. Much of what is known about Hfq function is drawn from studies of loss of function alleles of hfq in bacteria including Escherichia coli [v], Salmonella typhimurium [vi], and Vibrio cholerae [vii]. A common hfq mutant phenotype is deadening growth through exponential stage. All the same, loss of hfq office usually results in an array of mutant phenotypes, many of which are bacterium-specific. For example, East. coli hfq mutants showroom boring growth in vitro [5], survive poorly in stationary phase, and are sensitive to both H2O2 and hyperosmotic conditions [8]. In contrast, hfq mutants in Vibrio cholerae grow reasonably well in vitro (though they exhibit dumb growth in a mouse infection model), survive normally in stationary phase, and are fully resistant to both H2O2 and hyperosmotic conditions [seven]. Since many of the sRNAs that take been characterized require Hfq for their office, mayhap it is not surprising that loss of Hfq compromises a wide array of cellular processes. However, the fact that hfq mutations in different bacteria produce distinct phenotypes suggests distinct evolutionary roles for both Hfq and sRNAs in these divergent bacteria.
Shewanella oneidensis is a Gram-negative γ-Proteobacterium that is a facultative anaerobe establish in a wide range of environments. S. oneidensis is a member of a class of leaner known equally the dissimilatory metal-reducing leaner (DMRB). Under anaerobic weather condition, Southward. oneidensis has the power to utilize an impressively wide range of both organic and metal last electron acceptors. These metallic terminal electron acceptors include Cr(VI), Iron(III), Mn(Three) and (IV), and U(Vi) [9, 10]. The ability to mitigate the toxicity of soluble Cr(Six) and U(Six) by reduction to insoluble oxides of Cr(III) and U(IV), respectively, makes Shewanella an bonny potential bioremediating organism. In addition, the ability to deliver electrons to the extracellular surroundings allows Shewanella to generate electric electric current in microbial fuel cells [11]. Because the transition between aerobic and anaerobic metabolism is likely to occur frequently in nature, it is likely that sRNAs play a role in the transition between these metabolic states in S. oneidensis.
To gain insight into the functions of Hfq in South. oneidensis, we have constructed and characterized a nix allele of the hfq gene. The hfq∆ mutation in S. oneidensis is pleiotropic, resulting in defects in aerobic growth and greatly reduced recovery of colony forming units (CFU) from stationary stage cultures. In add-on, loss of hfq results in compromised anaerobic growth on fumarate and diminished capacity to reduce Cr(Half-dozen). Finally, nosotros have constitute that the South. oneidensis hfq∆ mutant is highly sensitive to oxidative stress. Chiefly, each of the hfq mutant phenotypes we have described is complemented by a plasmid-borne re-create of the wild type S. oneidensis hfq cistron, strongly suggesting that the mutant phenotypes we take observed are the result of the loss of hfq and not due to disruption of another gene. Our results advise that Hfq in Southward. oneidensis is involved in both common and organism-specific regulatory processes. To our noesis, this is the start characterization of an hfq mutant in a dissimilatory metal reducing bacterium.
Methods
Media and growth conditions
Aerobic cultures were grown in either LB (10g/Fifty tryptone, 5g/Fifty yeast extract, 10g/L NaCl) or a modified version of the original M1 medium [ix] with 30mM lactate as the electron donor. The modified M1 medium used in this study contains buffer/salts (3mM PIPES buffer, pH 7.0, 28mM NH4Cl, i.34mM KCl, iv.4mM NaHtwoPO4, 125mM NaCl), vitamins [81.8nM D-biotin (vitamin B7), 45.3nM folic acrid (vitamin Bix), 486.4nM pyridoxine HCl (vitamin Bhalf dozen), 132.8nM riboflavin (vitamin Btwo), 133.6nM thiamine HCl (vitamin B1), 406.2nM nicotinic acid (vitamin Biii), 209.8nM D-pantothenic acid, 0.74nM vitamin B12, 364.6nM p-aminobenzoic acid, 242.4nM lipoic acid], minerals [78.5μM nitriloacetic acrid (trisodium common salt), 249.1μM MgSO4 · 7 H2O, 29.6μM MnSO4 · one HtwoO, 171.1μM NaCl, 3.6μM FeSOiv · 7 HtwoO, 6.8μM CaCl2 · 2 H2O, 4.2μM CoCl2 · 6 H2O, 9.54μM ZnCl2, 0.4μM CuSO4 · five H2O, 0.21μM AlK(SO4)2 · 12 H2O, 1.61μM H3BO3, 1.24μM Na2MoOfour · 2 H2O, 1.01μM NiCl2 · 6 H2O, 0.76μM NaiiWOfour · ii H2O], and amino acids (135.9μM L-glutamic acid, 114.8μM 50-arginine, 190.3μM DL-serine). Anaerobic cultures were grown in modified M1 medium with 30mM lactate as the electron donor and 30mM sodium fumarate every bit the electron acceptor. Anaerobic conditions in broth cultures were accomplished by treating cultures in sealed test tubes using Oxyrase for Broth (Oxyrase, Inc., Mansfield, Ohio) as per the manufacturer's instructions.
All S. oneidensis cultures were grown at thirty°C, while Eastward. coli cultures were grown at 37°C. Cultures containing both Eastward. coli and S. oneidensis were grown at 30°C. Antibiotics were used at the following concentrations: Gentamicin (Gm): v μg/ml; Tetracycline (Tc): 10 μg/ml for E. coli; 1 μg/ml for S. oneidensis, [we used a lower concentration of Tc for choice of S. oneidensis than for E. coli considering we establish that the minimum inhibitory concentration (MIC) of Tc for S. oneidensis MR-i is <1 μg/ml (data non shown)]; Kanamycin (Km): 25 μg/ml; Ampicillin (Amp): 100 μg/ml.
For growth curves, 5ml LB Km cultures of South. oneidensis strains were inoculated from frozen permanent stocks and aerobically outgrown overnight (ten–12 hours). The overnight cultures were diluted in LB Km to an ABS600≅ 0.one or in modified M1 Km to an ABS600≅ 0.025 and aerobically outgrown to log stage (ABS600≅ 0.4-0.8). These exponentially growing cultures were then diluted to an ABS600≅ 0.one (LB Km) or to an ABS600≅ 0.025 (modified M1 Km). Aerobic cultures (xv-20ml) were grown in 125mL Erlenmeyer flasks shaken at 250RPM. Anaerobic cultures (15ml) were grown in sealed test tubes without shaking. Culture densities (ABS600) were monitored spectrophotometrically, and culture titers (CFU/ml) were determined by plating serial dilutions of cultures on LB Km plates.
Structure of the S. oneidensis hfq∆ mutant and hfqrescue construct
To generate a naught allele of hfq (So_0603 [12]) nosotros deleted most of the hfq open up reading frame and replaced it with a promoterless lacZ/gentamicin resistance gene cassette from pAB2001 [13]. We get-go PCR amplified a 5′ fragment using the primers GGCCCCGGGTAGAGCAAGGCTTTATTGATGAGGTAGC and GGCGCATGCGTCTTGTAAAGATTGCCCCTTAGCC and a iii' fragment using the primers GGCGCATGCACGATATGCCAAGTGGCGAATAAGG and GGCGGTACCAGCTCGTTGGGCGAAAATATCCAAAATCAG. Following restriction (restriction endonucleases purchased from New England Biolabs, Ipswich, MA) of the 5′ PCR fragment with XmaI and SphI and restriction of the 3' PCR fragment with SphI and KpnI, the two fragments were simultaneously ligated into pBSKS Ii + [14] that had been restricted with XmaI and KpnI. A 4.5kb SphI fragment from pAB2001 was then inserted into the SphI site of this plasmid to generate pBS-hfq∆. The XmaI-KpnI fragment from pBS-hfq∆, which contained the lacZ/gentamicin-disrupted hfq factor, was then cloned into XmaI/KpnI restricted pDMS197 [15], a R6K ori plasmid. The resulting plasmid, pDMS197-hfq∆ was transformed into East. coli SM10λpir [xvi], mated into S. oneidensis MR-i [9], and Gmr/Tcr single crossover recombinants were isolated. Post-obit growth in LB liquid without selection, cultures of these single crossovers were plated to LB plates containing Gm, 5% sucrose (west/v), and 0.one% NaCl (instead of omiting NaCl to increase the likelihood of isolating an hfq mutant in the consequence that loss of hfq resulted in cells sensitive to hypoosmotic atmospheric condition). Gmr Sucr Tcs hfq∆ mutant candidates were screened via PCR and DNA sequencing of the disrupted region. The sequence of the primers used for diagnostic PCR in Figure one are as follows: A (hfq 5' diagnostic) - ATAATGTGGTGCAATTTGCC; B (lacZ five' out) - CGTTGTAAAACGACGGGATCG; C (aacC1 3'out) - GATGCACTTTGATATCGACCC; D (hfq 3' diagnostic) - GAGTCCAACCACGCACTAGG.
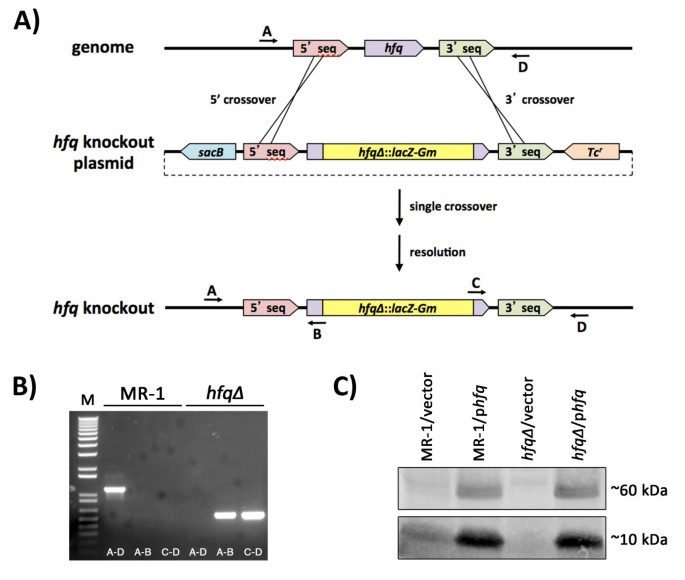
Construction and verification of a zippo allele of the Shewanella oneidensis MR-ane hfq gene. (A) Knockout strategy for the MR-ane hfq gene. Nigh of the hfq cistron coding sequence (all but the showtime 9 codons and last half-dozen codons) was replaced with a cassette containing a promoterless lacZ gene and a gentamicin resistance marker. (B) Agarose gel of analytical PCR reactions using wild type MR-1 (lanes 2–4) or hfq∆ mutant (lanes five–7) templates and primers A, B, C, and D (see Materials and Methods for primer sequences) indicated with arrows on the diagram in panel (A) The size standard (M) in lane 1 is 1kb plus DNA ladder (Invitrogen). (C) Western blot of SDS-PAGE-fractionated total poly peptide from various Shewanella strains probed with a polyclonal antibody raised against E. coli Hfq protein. Lanes 1 and 2: MR-1 containing pBBR1-MCS-2 (vector) or hfq rescue construct (phfq), respectively. Lanes 3 and four: hfq∆ containing vector or phfq, respectively. The antibody detects both putative Hfq monomers (~10kDa) likewise every bit putative Hfq homohexamers (~60kDa).
To generate an hfq rescue construct, we PCR amplified a 1.3kb genomic fragment containing the S. oneidensis MR-1 hfq coding sequence and ~1kb upstream of the hfq open reading frame. Based on hfq promoter analysis in E. coli, this fragment probable contains the native promoters for Southward. oneidensis hfq [17]. A PCR product was generated using the five' primer GGCAAGCTTCAGGAAAAACGGCTTTAGCTCTCG and the three' primer GGCGGTACCACTAAACCTTATTCGCCACTTGGC. Following brake with HindIII and KpnI, this PCR production was ligated to HindIII/KpnI restricted pBBR-1MCS2 [xviii]. The resulting plasmid, pBBR1-hfq, was transformed into E. coli S17-1λpir [19] and mated into S. oneidensis strains. In our easily, the pBBR1-MCS2 based vectors were stably maintained in S. oneidensis strains after xxx hours in LB Km cultures and after 120 hours in modified M1 Km cultures (information not shown).
Western blot analyses
3ml aliquots of Due south. oneidensis cultures were pelleted in a microcentrifuge for 2' at 20300 ten g. Bacterial pellets were frozen at −80°C, thawed, and then treated with Bacterial Protein Extraction Reagent (B-PER) in the presence of 100μg/ml lysozyme, 5U/ml DNAse I, and 1X Halt Protease Inhibitor Cocktail. Protein was quantified using the Pierce BCA Protein Assay Kit as per manufacturers instructions (all reagents were obtained from Thermo Scientific, Rockford, IL).
For western blot analysis, 90μg of protein per lane was size fractionated at 4°C using Whatsoever kD Mini-PROTEAN TGX Precast Gels (Bio-Rad, Hercules, CA). Proteins were then transferred to an Immobilon-PSQ PVDF membrane (EMD Millipore, Billerica, MA). Equivalent protein in dissimilar lanes was verified past Ponceau S staining of the membrane (data non shown). The membrane was blocked for 1 hr at room temperature using LI-COR Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE) and probed with a i:5000 dilution of primary antibody, rabbit anti-E. coli Hfq [20] overnight at four°C. The blot was done 4 times for 5 minutes each with PBS-T and then probed with a 1:10000 dilution of goat anti-rabbit secondary antibiotic conjugated to IRDye 800CW Infrared Dye (LI-COR Biosciences, Lincoln, NE) for 45 minutes at room temperature (~22°C). The absorb was done with PBS-T four times for 5 minutes each and then rinsed with PBS to remove residual Tween twenty. The absorb was and then imaged on a LI-COR Odyssey infrared scanner. Protein in Effigy 1C was harvested from 24 hr quondam LB Km cultures. Older cultures consistently accumulated higher levels of Hfq poly peptide, though our western blot results were consistent regardless of civilisation age at harvest; nosotros never observed Hfq protein in the hfq∆/empty vector cultures (Figure 1C and data not shown).
Chromium reduction assays
Chromium reduction assays were performed using a diphenylcarbazide-based quantitative, valence country specific, colorimetric assay for Cr(VI) [21]. Log phase cultures (ABS600≅ 0.5-0.viii) grown in modified M1 medium were diluted to ABS600≅ 0.4 in modified M1 medium that had been prewarmed to 30°C. The cultures were transferred to sealed test tubes and treated for xxx minutes at 30°C with Oxyrase for Broth (Oxyrase, Inc., Mansfield, Ohio) to remove oxygen. Following addition of 100μM KtwoCrO4, cultures were incubated without shaking in a 30°C h2o bath in sealed test tubes. 1ml aliquots of cultures were periodically removed and added to 13mm borosilicate glass tubes containing 0.25ml of a 0.v% diphenylcarbazide solution in acetone and ii.5ml 0.28N HCl. Following vortexing, ABS541 values for individual samples were measured in a SPECTRONIC 20D+ spectrophotometer (Thermo Scientific, Rockford, IL).
Oxidative stress assays
Overnight cultures grown in LB Km were diluted to an ABS600≅ 0.i. These cultures were outgrown for 2–iii hours to exponential stage (ABS600≅ 0.four-0.half-dozen) then diluted to an ABS600≅ 0.2. Following five minutes of aerobic growth, cultures were treated with H2O (mock), 0.4 mM H2O2 to induce peroxide stress, or five mM methyl-viologen (paraquat) to induce superoxide stress. Cultures were then grown aerobically for 15 minutes. Following treatment, each civilisation was serially diluted in triplicate in phosphate-buffered saline (PBS, pH 7.4). The dilutions were plated to LB Km plates within v minutes of harvest and grown overnight before scoring.
Results
Construction and verification of a null allele of hfq in Shewanella oneidensisMR-one
To study the roles played past the hfq cistron in Shewanella oneidensis, nosotros constructed a null allele of the putative hfq cistron (So_0603) in Southward. oneidensis strain MR-ane [ix, 12]. To disrupt the Due south. oneidensis hfq gene, we generated a knockout construct in which nosotros replaced most of the coding region of hfq with a cassette derived from pAB2001 [13] containing a promoterless lacZ cistron and a gentamicin resistance mark (Figure 1A - see Materials and Methods for details). This knockout fragment was cloned into the Tcr sacB-counterselectable R6K ori suicide vector pDMS197 [15] and mobilized into South. oneidensis MR-1. Single crossovers of the hfq knockout plasmid into the MR-one genome were isolated on the basis of both Gm resistance and power to abound on modified M1 defined medium. Following PCR verification, LB cultures of Gmr Tcr single crossovers were outgrown in LB medium without antibody selection and and so plated on LB agar containing Gm and 5% (west/v) sucrose. Elimination of the hfq gene in Sucr Tcsouth candidates was verified past PCR analyses (Figure 1B) and Dna sequencing analysis (data non shown). Western blotting demonstrated that the hfq∆ strain fails to produce Hfq poly peptide (Effigy 1C). Taken together, these data bespeak that we have generated a null allele of hfq in S. oneidensis.
The Shewanella oneidensis hfqmutant is defective in aerobic growth and exhibits reduced feasible cell counts in stationary phase
Considering mutations in the hfq gene compromise growth in many bacteria, nosotros analyzed the growth backdrop of the Southward. oneidensis hfq goose egg mutant. We characterized four strains: MR-1 containing pBBR1-MCS2 (hereafter referred to every bit empty vector), MR-1 containing pBBR1-hfq (pBBR1-MCS2 containing the wild type hfq factor under the command of its putative native promoter, time to come referred to as phfq), hfq∆ containing empty vector, and hfq∆ containing phfq. Loss of the hfq gene resulted in a pocket-sized colony phenotype on both LB agar plates (Effigy 2A) and modified M1 divers medium plates (data not shown). The small colony phenotype of the hfq mutant was completely rescued by phfq, but not past the empty vector lone (Figure 2A). The growth phenotype of wild type MR-i cells containing the phfq rescue plasmid was indistinguishable from MR-one cells containing the empty vector (Figure 2A), suggesting that additional, plasmid-borne copies of hfq that issue in college Hfq protein levels than plant in wild blazon cells (Figure 1C) do not significantly bear on the growth of S. oneidensis on solid media. Of note is that the hfq mutant colonies with empty vector never attain the same colony size as strains harboring wild type hfq, fifty-fifty after extended incubation (data not shown).
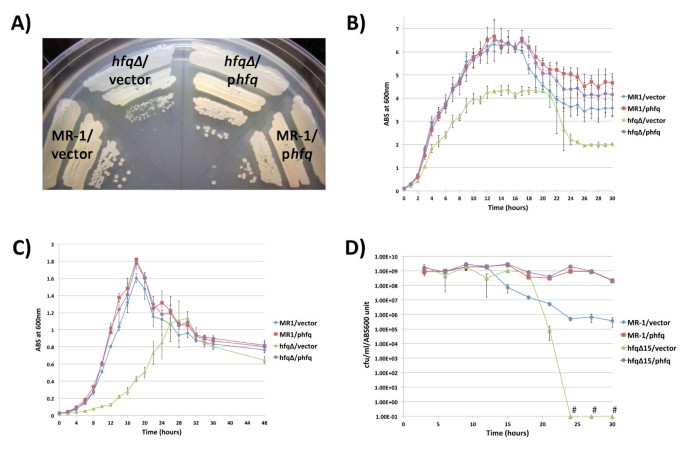
Shewanella oneidensis Hfq promotes exponential growth, last culture density, and viable prison cell counts in stationary phase. Growth of MR-i/empty vector, MR-1/phfq, hfq∆ /empty vector, and hfq∆ /phfq on LB agar containing kanamycin (A), in LB liquid containing kanamycin (B), or in modified M1 defined medium containing kanamycin (C). Plates in (A) were photographed later 24 hours of growth post-obit inoculation from frozen permanent stocks. 3 contained liquid cultures of each strain tracked in (B-D) were inoculated with log phase cultures grown in LB (B and D) or modified M1 medium (C). (D) Analysis of the human relationship between viable cell counts (CFU/ml) and civilization turbidity (ABS600) in LB cultures. Information points marked with "#" take CFU/ml/ABS600 values of nix. Error bars in panels (B-D) betoken a 99% confidence interval (P = 0.01).
To further characterize the nature of the growth defect in the hfq mutant, we compared the growth of the hfq mutant in aerobic liquid cultures to strains containing one or more wild type copies of the hfq gene (Figure 2B). When exponentially-growing cultures were diluted to late lag phase and outgrown beyond stationary phase, we consistently observed that the hfq∆/empty vector culture densities were significantly lower than those of the MR-1/empty vector cultures through exponential phase. In addition, the last cell densities of stationary phase hfq∆/empty vector cultures were significantly lower than the terminal cell densities of MR-1/empty vector cultures (Figure 2B). We likewise observed delayed growth during exponential stage and lower terminal stationary stage densities in hfq∆/empty vector liquid cultures grown in modified M1, a defined medium (Figure 2C). The growth and terminal density defects of the hfq mutant in liquid cultures were completely rescued by phfq, equally the growth of the hfq∆/phfq strain was indistinguishable from that of MR-1/empty vector in both LB (Figure 2B) and modified M1 (Figure 2C). Finally, extra copies of hfq that upshot in higher Hfq protein levels (Effigy 1C) do not appear to alter the growth of S. oneidensis in liquid medium, as growth of MR-1/phfq and hfq∆/phfq cultures was indistinguishable from that of MR-ane/empty vector cultures in LB and modified M1 media (Figures 2B and 2C).
To determine whether the relationships between spectrophotometric measurements of culture density and jail cell number were comparable betwixt the strains used in our study, we determined the relationship betwixt ABS600 values and viable cell counts for MR-1/empty vector, MR-one/phfq, hfq∆/empty vector, and hfq∆/phfq at various times during culture outgrowth. In both LB cultures (Figure 2D) and modified M1 cultures (data not shown), the relationship between ABS600 and colony forming units per ml (CFU/ml) was consistent for all iv strains throughout exponential phase and until approximately mid-stationary phase. This indicates that the optical properties of the four strains characterized are highly similar at 600nm and that turbidity measurements are an accurate indicator of culture growth until mid stationary phase.
Intriguingly, we observed that the CFU/ml/ABS600 values for the four strains used in our studies diverged dramatically post-obit mid-stationary stage (Figure 2nd). Nosotros consistently found that hfq∆/empty vector cultures experienced a precipitous drop in CFU counts late in stationary phase. In most cases, culturable cell counts had dropped to nix CFU/ml by 30 hours. In contrast, MR-1/empty vector cultures were much more robust than hfq∆ /empty vector cultures, maintaining significant CFU counts, fifty-fifty after xxx hours of growth. The information presented in Figure 2D represents a typical issue for an iteration of this experiment. It is worth noting, however, that the timing of the starting time of the reduction in CFU counts observed for the MR-1/empty vector strain and for the hfq∆/empty vector strain could vary by several hours betwixt contained cultures, even parallel cultures simultaneously inoculated using the same preculture (data non shown). Furthermore, we also consistently observed that MR-i/phfq and hfq∆/phfq cultures, which contain more Hfq protein than wild type cultures at 24 hours (Figure 1C), retained significantly college numbers of colony forming units compared to MR-1/empty vector cultures in extended stationary stage. Taken together, our loss-of-part and gain-of-role analyses demonstrate that Hfq promotes prison cell survival or culturability in extended stationary phase.
The hfq∆mutant is impaired in anaerobic growth and chromium reduction
To characterize the role of S. oneidensis hfq in anaerobic growth, we compared the growth kinetics of strains MR-1/empty vector, MR-i/phfq, hfq∆/empty vector, and hfq∆ /phfq grown in modified M1 defined medium with fumarate as the terminal electron acceptor. Similar to the growth defects observed during aerobic growth, anaerobic hfq∆ /empty vector cultures grew more than slowly during exponential phase and reached a lower terminal density than MR-i/empty vector cultures. (Figure 3A). The growth and terminal density defects of hfq mutant cultures in anaerobic modified M1 plus fumarate were completely rescued by phfq, as the growth of the hfq∆/phfq strain was indistinguishable from that of MR-ane/empty vector (Effigy 3A). Extra copies of hfq did not alter the ability of South. oneidensis to utilize fumarate equally a last electron acceptor, every bit growth of MR-1/phfq and hfq∆/phfq cultures was very like to that of MR-1/empty vector cultures (Figure 3A).
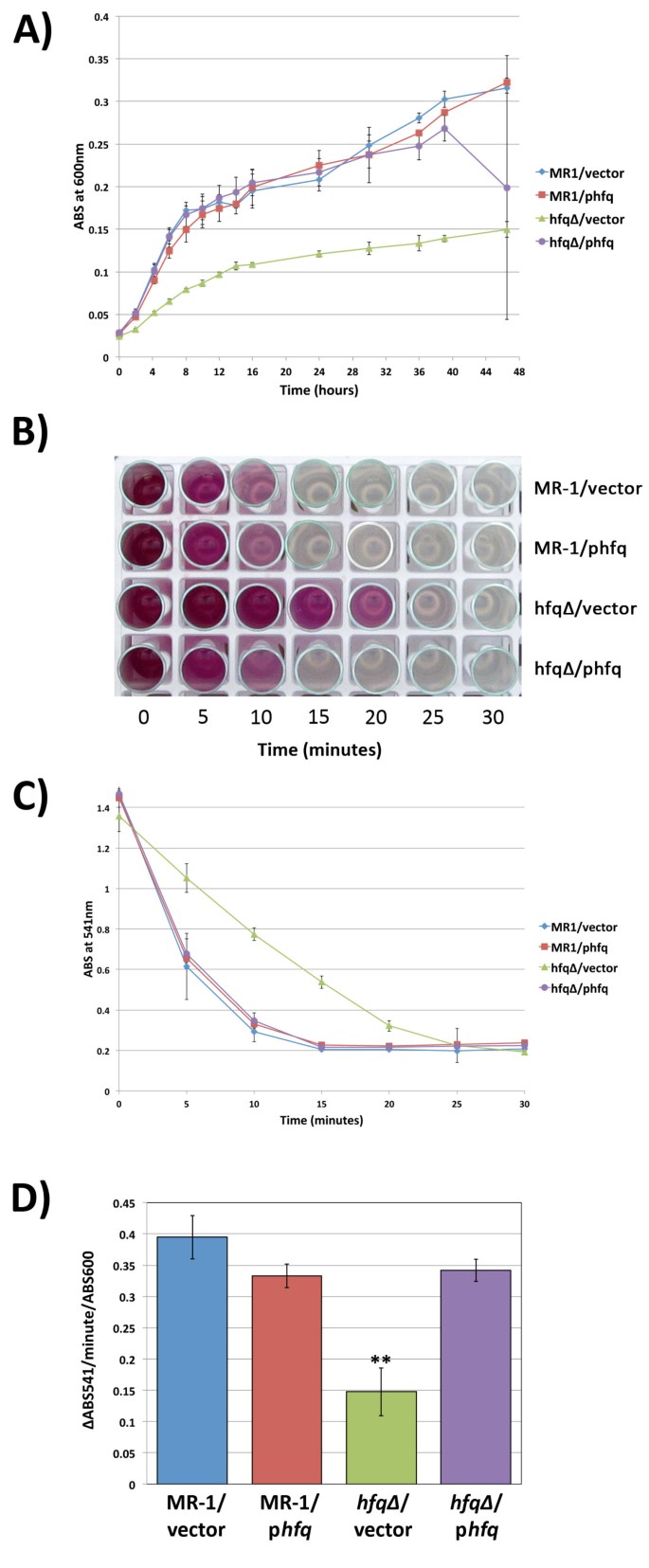
The hfq∆ mutant is deficient in anaerobic respiration. (A) Growth of MR-1/empty vector, MR-1/phfq, hfq∆ /empty vector, and hfq∆ /phfq under anaerobic conditions with fumarate equally the terminal electron acceptor. Data presented is from three independent cultures. Mistake bars represent a 99% conviction interval (P = 0.01). (B and C) Results of chromium reduction assays. Chromium reduction/disappearance of Cr(VI) was assayed using the diphenylcarbazide method. Error bars represent a 99% confidence interval (P = 0.01). (D) Quantification of the rate of Cr(VI) reduction (expressed as the change in ABS541 per minute per ABS600) in the cultures tracked in (C) above during the first five minutes following the addition of chromium to anaerobic cultures. Error bars represent the standard divergence for triplicate cultures. ** indicates that the hfq∆ /empty vector charge per unit is statistically different from the other three strains (P < 0.002 for all three comparison in unpaired 2-tailed Student'south T-tests).
To determine whether loss of hfq altered the ability of South. oneidensis to utilise chromium every bit a terminal electron acceptor, we measured the kinetics of Cr(VI) reduction past our four hfq strains using diphenylcarbazide, a reagent that binds to Cr(Half dozen) and produces a regal color proportional to the amount of Cr(VI) in the sample [21]. In fully anaerobic cultures with no other electron acceptor nowadays, metal reduction begins immediately upon addition of Cr(Half dozen), and the rate of reduction is highest in the first five minutes post-obit Cr(6) addition. As the Cr(Half dozen) is reduced, the assay results keep from a deep regal color at early timepoints to a colorless solution at later timepoints, allowing quantification of the disappearance of Cr(VI) (Figure 3B). In our assays, the ABS541 values for the assay timepoints do not fall below ~0.2 because of the absorbance contribution of the cells at 541nm (data not shown). Though all strains tested eventually reduced all of the detectable Cr(VI), we found that the hfq∆ mutant is significantly slower in reducing Cr(VI) and takes almost twice as long to employ all bachelor Cr(6) as strains containing wild blazon hfq (Figures 3B and 3C). In addition, the rate of Cr(VI) reduction (∆ABS541) per infinitesimal per ABS600 unit of measurement during the first 5 minutes of metal reduction for the hfq∆/empty vector strain was less than half that of strains containing at to the lowest degree one re-create of wild type hfq (Figure 3D).
To be sure that the Cr(Six) reduction defect observed in the hfq∆/empty vector strain was due to a defect in metal reduction and not expiry of cells due to an increased sensitivity to Cr, nosotros measured the CFU/ml present in cultures of all four strains both before and after the 30 minute chromium reduction analysis. We found no meaning differences in the CFU/ml values measured before and after the assay for any of the four strains used in our experiments (data not shown). As observed in our growth analyses higher up, the CFU/ml/ABS600 values for the four anaerobic strains did not vary significantly among the cultures (data not shown), demonstrating again that turbidity measurements were an accurate reflection of viable prison cell counts. Taken together, our results suggest that hfq∆/empty vector cells have an intrinsic defect in use of chromium as a terminal electron acceptor during anaerobic respiration.
The hfq∆mutant is highly sensitive to oxidative stress
Mutations in hfq in Eastward. coli result in an increased sensitivity to oxidative stress in addition to poor survival in stationary phase [8]. Given the poor survival of the S. oneidensis hfq∆ mutant in extended stationary phase, a catamenia typically characterized by increased oxidative stress [22, 23], we decided to explore the ability of the hfq∆ mutant to cope with oxidative stress. Exponentially growing cultures of MR-1/empty vector, MR-1/phfq, hfq∆/empty vector, and hfq∆/phfq were treated with either HiiO2 to induce peroxide stress or methyl viologen to induce superoxide stress. Serial dilutions of these cultures were and so plated, and the survival rates relative to mock (H2O) treated cultures were measured. The survivorship of each strain was determined past computing the ratio of viable cells in the treated cultures to viable cells in the mock treated cultures. Strains with a wild type re-create of hfq survived significantly better than the hfq∆/empty vector strain when challenged with either H2Oii (Figure 4A and 4B) or methyl viologen (Effigy 4C and 4D). These data suggest that one function of S. oneidensis Hfq is to protect cells against oxidative stress.
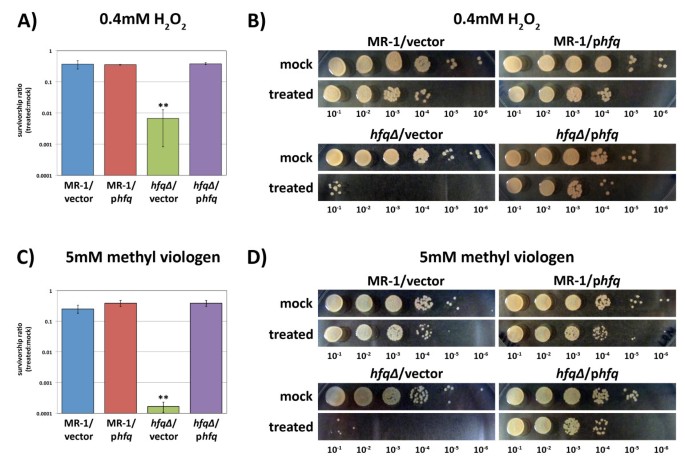
The hfq∆ mutant is highly sensitive to oxidative stress. Aerobic, exponentially growing cultures of MR-1/empty vector, MR-1/phfq, hfq∆ /empty vector, and hfq∆ /phfq were treated for 15 minutes with either (A and B) 0.iv mM HiiO2 or (C and D) 5mM methyl viologen (paraquat) and then immediately titered. Survivorship ratios were adamant by calculating the ratio of the number of feasible cells in the treated cultures to the number of viable cells in mock (H2O) treated cultures. Values on the graphs are the mean survivorship ratios for three independent experiments. Error bars in (A) and (C) indicate standard deviations. The hfq∆ /empty vector survival rate is statistically different from the other 3 strains in both the H2O2 and methyl viologen experiments (** indicates that P < 0.005 for comparison of the hfq∆ /empty vector strain information to each of the other strains in unpaired ii-tailed Pupil'south T-tests). Panels (B) and (D) demonstrate typical ten-fold dilution series results obtained after treatment of strains MR-1/empty vector, MR-ane/phfq, hfq∆ /empty vector, and hfq∆ /phfq with (B) HiiO (mock) or H2Otwo or (D) HiiO (mock) or methyl viologen.
Word and conclusions
In this paper, we describe the construction and characterization of a nothing allele of the hfq factor in the bacterium Southward. oneidensis. Loss of the hfq gene produces an array of phenotypes, each of which is fully complemented by an exogenously supplied re-create of the wild blazon hfq gene. To our knowledge, this is the start report of an hfq gene knockout in a dissimilatory metal reducing bacterium. Given the varied roles played by Hfq in diverse leaner, we expect that this mutant volition exist both a useful tool for analyzing sRNA part in South. oneidensis likewise as for understanding Hfq role in full general.
Information technology is clear from our analyses that S. oneidensis Hfq positively regulates exponential phase growth. The exponential stage growth defect of the hfq mutant is non growth medium specific, equally we observe dull exponential stage growth in both complex and defined media. In addition, we observe this defect when cells are grown under both aerobic and anaerobic conditions. It is not yet articulate why the hfq mutant grows slowly when nutrients are plentiful. It is possible that the hfq mutant growth phenotype is a outcome of a defect in food conquering, a possibility suggested by the fact that hfq mutants in a variety of bacteria limited lower levels of genes involved in food uptake [6, 24–26]. It is besides possible that the hfq mutant has more general set of metabolic defects that underlie its slow growth phenotype, which may explicate why the hfq mutant is less efficient in Cr(VI) reduction. Alternatively, hfq may accept a more specific role in utilization of Cr(VI) as a terminal electron acceptor.
A second notable hfq mutant growth phenotype is the failure of mutant cultures to reach a terminal cell density equally high as those seen in wild type cultures. Though it is non however clear what underlies this mutant phenotype, it is possible that the hfq mutant is unable to fully utilize the available nutrients in the medium or that it exhausts a nutrient that is rate limiting for growth more than apace than wild type cells. Alternatively, the hfq mutant may produce more of, or exist more sensitive to, at to the lowest degree 1 growth-suppressing product produced during Due south. oneidensis growth.
Strikingly, S. oneidensis hfq mutant cultures exhibit a astringent loss of colony forming units in stationary phase, with cultures frequently displaying no detectable CFU. One possibility is that Hfq promotes cell survival in stationary phase, and thus loss of hfq results in loss of civilisation viability. An alternative explanation is that Hfq functions to prevent cells from entering a feasible but not culturable (VBNC) land [27], and thus reduced CFU/ml counts in hfq∆ mutant cultures are due to hfq∆ cells precociously assuming VBNC status. Both of these models are supported past the fact that moderate overexpression of Hfq results in higher CFU/ml counts during stationary phase when compared to cells with wild blazon Hfq poly peptide levels. Farther experimentation will exist required to differentiate between these ii possible explanations for the greatly reduced CFU/ml counts in hfq∆ stationary phase cultures.
Because the hfq mutant is highly sensitive to oxidative stress, it is possible that the stationary stage survival defect in hfq mutant cells is a consequence of poor resistance to oxidative stress. Multiple Hfq-dependent sRNAs (arcZ, dsrA, and rprA) positively regulate expression of the stationary phase sigma cistron RpoS in other systems [28–30]. Thus, information technology is possible that loss of Hfq in S. oneidensis causes depression rpoS expression, resulting in poor induction of the rpoS regulon. Lower rpoS regulon induction may increment the oxidative stress sensitivity of the hfq mutant and consequently reduce stationary phase survival. Some other possibility that remains to exist explored is whether the hfq mutant's sensitivity to oxidative stress is due to altered function of superoxide dismutase (sodB – So_2881) and/or one or more than of the 4 genes predicted to encode proteins with catalase activity katB (So_1070), So_1771.ii, katG2 (So_4405), and katG1 (So_0725)] [12]. Finally, it will be of involvement to decide whether S. oneidensis contains an hfq-dependent OxyR-OxyS organization that is involved in response to oxidative stress as in other systems [20, 31].
We are currently investigating the mechanisms past which S. oneidensis Hfq promotes growth, last culture density, and stationary phase survival. However, given that Hfq has been broadly implicated in the function of many sRNAs in other systems [32], the S. oneidensis hfq mutant generated in this study will facilitate analysis of the roles of Hfq and sRNAs in adaptation to a wide range of environmental conditions. This is of particular interest since a previous report demonstrated that S. oneidensis sRNAs do non always take completely overlapping functions with their homologs in other systems [33].
References
-
Geissmann TA, Touati D: Hfq, a new chaperoning role: bounden to messenger RNA determines admission for small RNA regulator. EMBO J. 2004, 23 (2): 396-405. 10.1038/sj.emboj.7600058.
-
Gottesman Southward: The pocket-size RNA regulators of Escherichia coli: roles and mechanisms. Annu Rev Microbiol. 2004, 58: 303-328. x.1146/annurev.micro.58.030603.123841.
-
Moller T, Franch T, Hojrup P, Keene DR, Bachinger HP, Brennan RG, Valentin-Hansen P: Hfq: a bacterial Sm-like poly peptide that mediates RNA-RNA interaction. Mol Cell. 2002, ix (1): 23-xxx. x.1016/S1097-2765(01)00436-ane.
-
Panja S, Woodson SA: Hexamer to monomer equilibrium of E. coli Hfq in solution and its bear upon on RNA annealing. J Mol Biol. 2012, 417 (5): 406-412. 10.1016/j.jmb.2012.02.009.
-
Tsui HC, Leung HC, Winkler ME: Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli G-12. Mol Microbiol. 1994, 13 (ane): 35-49. 10.1111/j.1365-2958.1994.tb00400.ten.
-
Sittka A, Pfeiffer V, Tedin M, Vogel J: The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol. 2007, 63 (1): 193-217. 10.1111/j.1365-2958.2006.05489.10.
-
Ding Y, Davis BM, Waldor MK: Hfq is essential for Vibrio cholerae virulence and downregulates sigma expression. Mol Microbiol. 2004, 53 (1): 345-354. x.1111/j.1365-2958.2004.04142.ten.
-
Muffler A, Traulsen DD, Fischer D, Lange R, Hengge-Aronis R: The RNA-binding poly peptide HF-I plays a global regulatory role which is largely, just not exclusively, due to its function in expression of the sigmaS subunit of RNA polymerase in Escherichia coli. J Bacteriol. 1997, 179 (1): 297-300.
-
Myers CR, Nealson KH: Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988, 240 (4857): 1319-1321. 10.1126/science.240.4857.1319.
-
Nealson KH, Saffarini D: Iron and manganese in anaerobic respiration: ecology significance, physiology, and regulation. Annu Rev Microbiol. 1994, 48: 311-343. 10.1146/annurev.mi.48.100194.001523.
-
Lovley DR: Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol. 2006, iv (7): 497-508. x.1038/nrmicro1442.
-
Heidelberg JF, Paulsen It, Nelson KE, Gaidos EJ, Nelson WC, Read TD, Eisen JA, Seshadri R, Ward N, Methe B, et al: Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat Biotechnol. 2002, twenty (11): 1118-1123. ten.1038/nbt749.
-
Becker A, Schmidt Yard, Jager West, Puhler A: New gentamicin-resistance and lacZ promoter-probe cassettes suitable for insertion mutagenesis and generation of transcriptional fusions. Cistron. 1995, 162 (i): 37-39. 10.1016/0378-1119(95)00313-U.
-
Alting-Mees MA, Short JM: pBluescript II: gene mapping vectors. Nucleic Acids Res. 1989, 17 (22): 9494-10.1093/nar/17.22.9494.
-
Edwards RA, Keller LH, Schifferli DM: Improved allelic substitution vectors and their use to analyze 987P fimbria gene expression. Gene. 1998, 207 (2): 149-157. 10.1016/S0378-1119(97)00619-7.
-
Miller VL, Mekalanos JJ: A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988, 170 (6): 2575-2583.
-
Tsui HC, Feng One thousand, Winkler ME: Transcription of the mutL repair, miaA tRNA modification, hfq pleiotropic regulator, and hflA region protease genes of Escherichia coli K-12 from clustered Esigma32-specific promoters during heat shock. J Bacteriol. 1996, 178 (xix): 5719-5731.
-
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM: Iv new derivatives of the broad-host-range cloning vector pBBR1MCS, conveying dissimilar antibiotic-resistance cassettes. Cistron. 1995, 166 (1): 175-176. 10.1016/0378-1119(95)00584-i.
-
Simon R, Priefer U, Puhler A: A broad host range mobilization system for in vivo genetic technology: transposon mutagenesis in gram negative bacteria. Nat Biotech. 1983, 1: 784-791. 10.1038/nbt1183-784.
-
Zhang A, Wassarman KM, Ortega J, Steven Air conditioning, Storz G: The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol Cell. 2002, 9 (ane): 11-22. 10.1016/S1097-2765(01)00437-three.
-
Urone PF: Stability of colorimetric reagent for chromium, s-diphenylcarbazide, in various solvents. Anal Chem. 1955, 27: 1354-1355. 10.1021/ac60104a048.
-
Dukan S, Nystrom T: Bacterial senescence: stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental consecration of the heat stupor regulon. Genes Dev. 1998, 12 (21): 3431-3441. 10.1101/gad.12.21.3431.
-
Navarro Llorens JM, Tormo A, Martinez-Garcia Due east: Stationary phase in gram-negative leaner. FEMS Microbiol Rev. 2010, 34 (four): 476-495. 10.1111/j.1574-6976.2010.00213.x.
-
Geng J, Song Y, Yang L, Feng Y, Qiu Y, Li Yard, Guo J, Bi Y, Qu Y, Wang W, et al: Involvement of the post-transcriptional regulator Hfq in Yersinia pestis virulence. PLoS Ane. 2009, 4 (7): e6213-x.1371/journal.pone.0006213.
-
Guisbert Due east, Rhodius VA, Ahuja Due north, Witkin East, Gross CA: Hfq modulates the sigmaE-mediated envelope stress response and the sigma32-mediated cytoplasmic stress response in Escherichia coli. J Bacteriol. 2007, 189 (5): 1963-1973. 10.1128/JB.01243-06.
-
Sonnleitner E, Schuster K, Sorger-Domenigg T, Greenberg EP, Blasi U: Hfq-dependent alterations of the transcriptome profile and effects on quorum sensing in Pseudomonas aeruginosa. Mol Microbiol. 2006, 59 (5): 1542-1558. 10.1111/j.1365-2958.2006.05032.x.
-
Oliver JD: The viable but nonculturable state in bacteria. J Microbiol. 2005, 43 (Spec No): 93-100.
-
Lease RA, Cusick ME, Belfort Thou: Riboregulation in Escherichia coli: DsrA RNA acts past RNA:RNA interactions at multiple loci. Proc Natl Acad Sci U.s.. 1998, 95 (21): 12456-12461. ten.1073/pnas.95.21.12456.
-
Majdalani Northward, Cunning C, Sledjeski D, Elliott T, Gottesman S: DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc Natl Acad Sci U.s.a.. 1998, 95 (21): 12462-12467. 10.1073/pnas.95.21.12462.
-
Majdalani N, Hernandez D, Gottesman S: Regulation and mode of activeness of the second small RNA activator of RpoS translation, RprA. Mol Microbiol. 2002, 46 (3): 813-826. 10.1046/j.1365-2958.2002.03203.x.
-
Zhang A, Altuvia South, Tiwari A, Argaman L, Hengge-Aronis R, Storz G: The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) poly peptide. EMBO J. 1998, 17 (twenty): 6061-6068. 10.1093/emboj/17.20.6061.
-
Vogel J, Luisi BF: Hfq and its constellation of RNA. Nat Rev Microbiol. 2011, 9 (viii): 578-589. 10.1038/nrmicro2615.
-
Yang Y, McCue LA, Parsons AB, Feng S, Zhou J: The tricarboxylic acid cycle in Shewanella oneidensis is contained of Fur and RyhB control. BMC Microbiol. 2010, 10: 264-10.1186/1471-2180-x-264.
Acknowledgements
Nosotros give thanks Aixia Zhang for supplying the anti-Hfq antibody. Thanks to Fr. Nicanor Austriaco, O.P. and Jennifer Gervais for thoughtful discussions and critical reading of the manuscript. Research reported in this publication was supported past an Institutional Development Accolade (Thought) from the National Found of General Medical Sciences of the National Institutes of Wellness under grant number eight P20 GM103430-12. Additional funding was provided by a Providence College Undergraduate Enquiry Grant to CMB and an American Society for Microbiology (ASM) Summer Research Fellowship to MTG.
Author data
Affiliations
Corresponding author
Additional data
Authors' contributions
BJP and CMB conceived of and designed all the experiments in the newspaper, executed experiments, collected and interpreted the data, and drafted the manuscript. Strain construction and verification was performed by BJP, CMB, MLK, TMH, NQM, JMO, KED, MTG, TM, and ZS. BJP and CMB performed stationary stage survival assays and metal reduction assays. BJP, CMB, TMH, MLK, MTG, and NQM designed and performed oxidative stress experiments. All authors read and approved the final manuscript.
Authors' original submitted files for images
Rights and permissions
Open Access This article is published nether license to BioMed Key Ltd. This is an Open Access article is distributed under the terms of the Artistic Eatables Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and Permissions
Almost this commodity
Cite this article
Brennan, C.M., Keane, M.Fifty., Hunt, T.Grand. et al. Shewanella oneidensisHfq promotes exponential phase growth, stationary stage culture density, and prison cell survival. BMC Microbiol 13, 33 (2013). https://doi.org/ten.1186/1471-2180-13-33
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/1471-2180-13-33
Keywords
- Shewanella oneidensis
- Hfq
- Metal reduction
- Oxidative stress
- Stationary phase survival
Source: https://bmcmicrobiol.biomedcentral.com/articles/10.1186/1471-2180-13-33
0 Response to "In a Sealed Test Tube After the Fourth Phase Growth the Cell Number Increased Again Because"
Postar um comentário